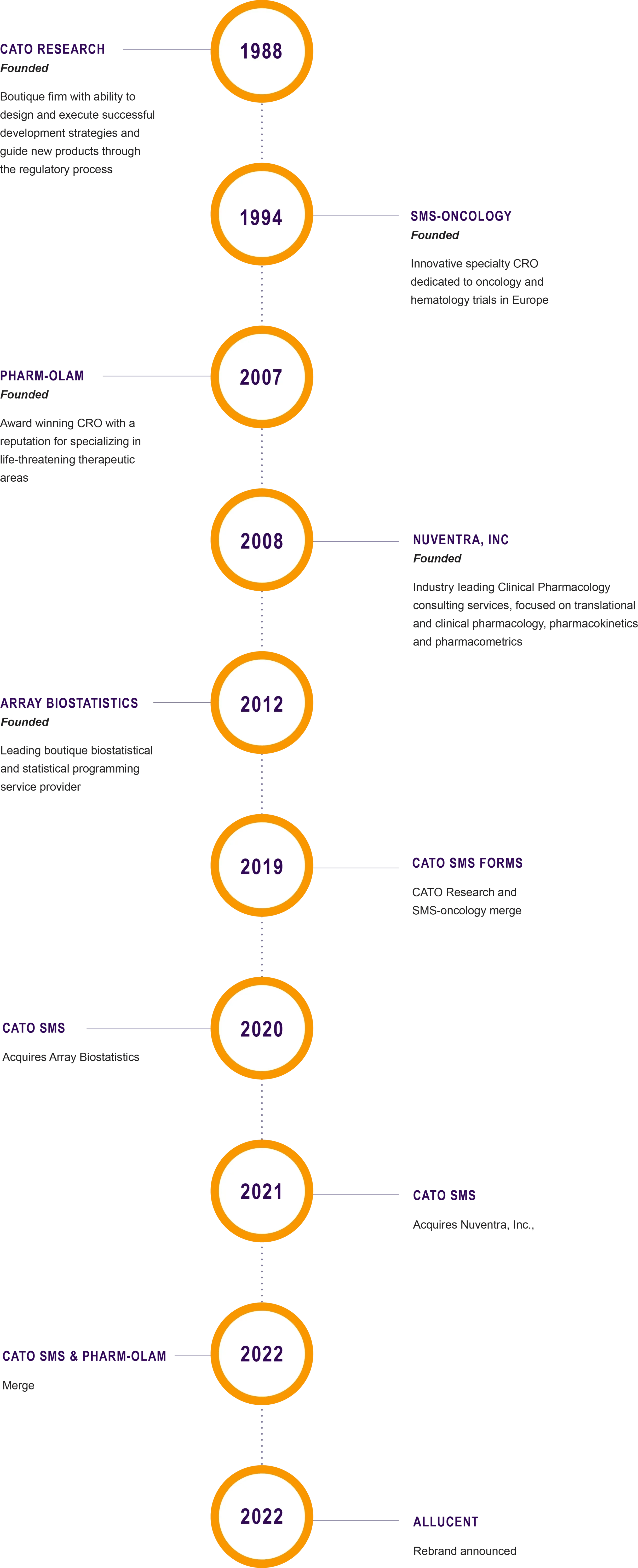
Company Origins
In the spring of 2022, Allucent came into being with the merger of several high-performance companies serving the specialized needs of small and mid-size biopharma. Derived from the Latin verb alluceo, Allucent means to shine a light upon something or to create an opportunity. With a new name and bold branding, Allucent united the company and its employees under one purpose: putting the spotlight on biotech innovators and helping them deliver treatments to patients with unmet needs.
OUR CORPORATE TIMELINE