Because rare diseases affect a small population of people who are dispersed across the world, it makes country and site selection, patient identification, clinical trial recruitment and long-term retention particularly complex. Furthermore, many rare diseases are difficult to diagnose — in some cases they have been discovered only recently with medicine advancements and development of new diagnostic techniques. Obtaining an accurate diagnosis is often one of the first obstacles to overcome.
From a clinical research point of view, rare disease studies are usually expected to have slow recruitment with a small number of patients recruited per site and with a typically low number of study participants overall. This poses specific challenges that you must account for in your study planning and site selection strategy.
Patient Eligibility Screening: A balance between tradition and innovation
Patient identification and recruitment challenges are nothing new in rare disease clinical trials. Navigating the complexities of rare diseases often requires a delicate balance between tradition and innovation and we must not overlook the enduring value of well-proven, traditional methods which have served as the foundation for patient eligibility screening.
The advent and utilisation of AI and machine learning to leverage real-world evidence and genetic powered data is new and on the increase. By accessing available patient data we are able to apply advanced genome and exome sequencing to predict disease causing genes and mutations across variable rare disease datasets accelerating diagnosis accuracy.
Once diagnosis has taken place Allucent maximises the recruitment potential of those patients identified. To help minimize high rates of both screen failures and protocol deviations Allucent has developed and refined a subject eligibility verification process which incorporates potential patients identified through investigator databases, referrals, patient advocacy groups, and various other recruitment strategies, into a central database known as a Screening Navigator Tool.
Allucent Screening Navigator Tool – Virtual Waiting Room
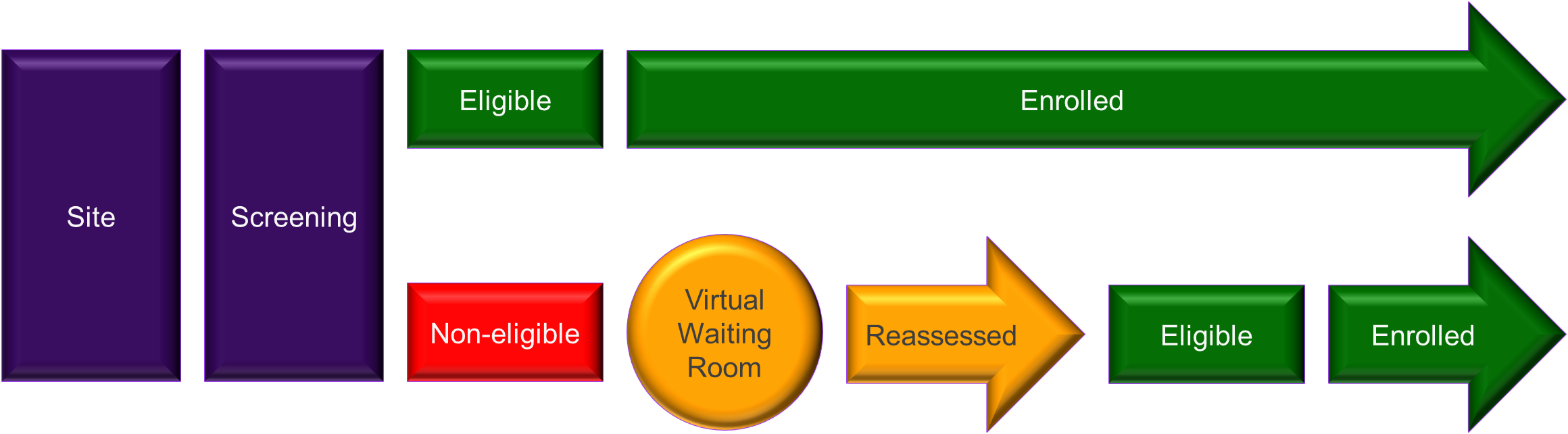
Once entered into the platform these patients undergo a thorough screening against targeted eligibility criteria, accompanied by supplementary information. Our Medical Monitors review the curated data and assess the eligibility of each candidate and provide invaluable feedback to the respective investigators. Patients deemed ineligible during the initial screening find themselves in a “Virtual Waiting Room”, a strategic feature designed for periodic reassessment. The frequency of the reassessments is tailored to the unique nature of the disease, individual patient characteristics, and the study requirements.
Patients residing in the virtual waiting room receive regular updates and informational resources while undergoing periodic evaluation. This iterative process not only serves as a dynamic source of pre-identified patients for current trails but also positions them for potential inclusion in future clinical developments.
Investing Time and Effort Early will be Rewarded
Allucent’s Screening Navigator Tool delivers a source of potentially eligible patients contributing significantly to the success or our rare disease trials both in the present and in the trajectory of future clinical studies. To date, the efficacy of the tool has demonstrated remarkable success in both hematologic and endocrine rare disease clinical trials diminishing screen failure rates from between 25% – 75% and protocol deviations down to 0% for patient inclusion in a previous trial.
While it’s true that clinical trials for rare diseases present a unique set of obstacles, they can be overcome and throughout all your preparations, keep patient-centricity at the forefront so as not to add further burdens on to your often-fragile study population.
Case Study: Learn how Allucent overcame three core operational challenges from a recent rare disease phase III trial.
