Site location can have a significant impact on clinical trial success. Study protocols often have established timelines and budget restrictions that offer little room for flexibility. There are also different regulations and regulatory timelines that have to be considered and managed – and that’s when there isn’t a pandemic. The COVID-19 Crisis has forced many researchers to institute different study adaptions to keep research going, such as prioritizing sites that have been least impacted by COVID-19.
With so many moving parts, site selection for clinical trials can become very regional. Researchers develop relationships with local investigators, a strong understanding of how to meet regulations, and overall best practices for study success within a given area. However, the variables that make a site optimal do shift over time. Responsible researchers have to regularly evaluate whether specific locations still work as well today as they once did.
The Central and Eastern Europe (CEE) region is a good example. It has a long history of being an optimal location for a variety of trials, oncology included. In a recent talk, Pharm-Olam’s Executive Medical Director Oncology Lead Europe, Ben Manderman, discussed whether it is still worth doing clinical trials in the CEE. In this article, we will review some of those findings.
Countries in CEE
Let’s start by defining what we mean when we refer to CEE. The area is unique in that some of the countries in the region belong to the European Union while others do not. We categorize countries as being part of the CEE on the basis of whether they share certain characteristics.
Demographics
Within Central and Eastern Europe, demographics are very diverse. Whereas some countries have large populations, the region also includes smaller countries with lower numbers of people. Age distributions also vary, with some countries, like Kazakhstan, having a relatively young population (over 70% are under the age of 45) and others, such as Lithuania, being more evenly split (50% are under the age of 45). People within the CEE also tend to be very diverse with regard to alcohol consumption, a vital consideration when studying alcohol-related conditions, and smoking prevalence.
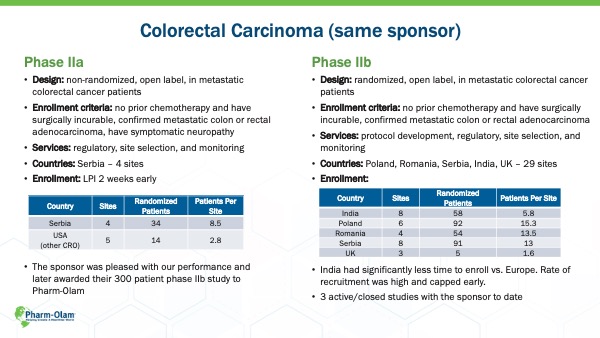
CASE STUDY: Colorectal Carcinoma
Patient Access
As diverse as people within CEE can be, one important commonality is that patient access is favorable for researchers. CEE citizens are frequently concentrated around population centers – and this helps improve patient access. Transportation is often readily available (reducing an important barrier to participation for many people) and advanced medical resources are easier to come by. Population centers often have comprehensive medical centers, screening equipment, lab services, and more that may be more difficult to access in rural areas.
Furthermore, many CEE countries have centralized healthcare in place. This fact means that there is an established referral system and concentrated pools of qualified investigators as well as a well-maintained database of patients with specific conditions. Several cities in CEE have large oncology centers too, including the Marie Sklodowska Curie Memorial Cancer Centre and Institute of Oncology (MCMCC) in Warsaw and the N.N. Blokhin Russian Cancer Research Center in Moscow.
Centralized medicine also contributes to successful enrollment in countries with a national healthcare system for other reasons. In as much as treatment can be guaranteed to the entire population of the country, that standard of care can be drastically different from other healthcare systems. Funding is limited, prohibiting access to more expensive treatment options and modern therapies. In turn, patients are frequently willing to participate in clinical research to gain access to new therapeutics.
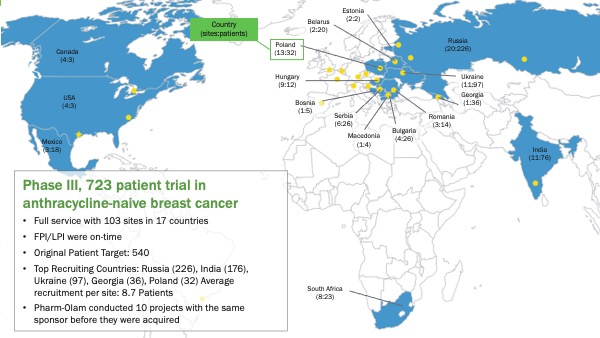
CASE STUDY: Phase III, 723 patient trial in anthracycline-naive breast cancer
Regulatory Timelines
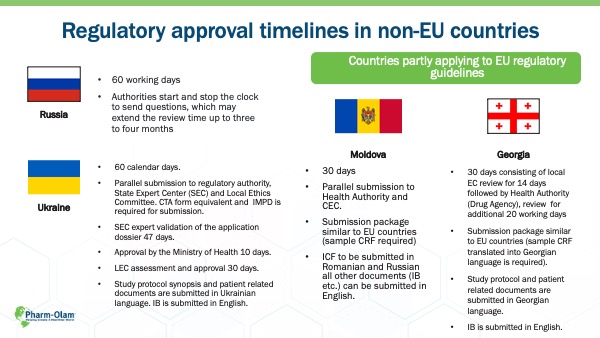
Regulatory timelines also benefit clinical trials. The average time to clinical trial registration can be as long as 180 days for EU members, including CEE countries, but non-EU CEE countries can see timelines as short as 30 days. That difference can be significant when Sponsors are working to get a drug to market as quickly as possible.
CASE STUDY: EU Timelines vs CEE Timelines
Quality Assurance
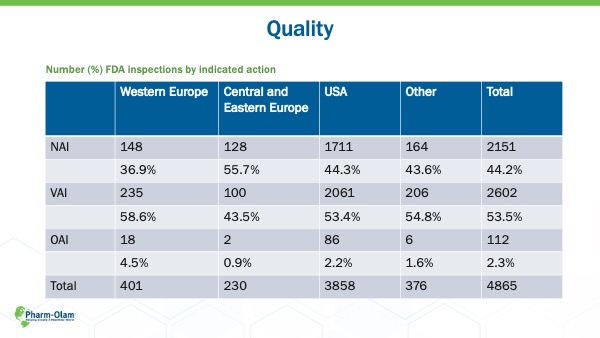
With such quick regulatory timelines and, in some cases, lower economic development, CEE countries can sometimes be disregarded. Opponents to the approach of choosing CEE for clinical trial site locations will sometimes site quality as a concern. However, in Pharm-Olam’s experience, that claim is utterly unfounded. The percentage of studies with no action indicated by the FDA are significantly better than Western Europe, the USA, or other parts of the world.
CASE STUDY: FDA INSPECTION RESULTS
Why is CEE Attractive for Clinical Research?
Central and Eastern Europe has been a veritable mecca for clinical research in recent years, particularly with regard to cancer studies – and there are several reasons for the region’s popularity. Oncology trials require a certain mix of demographics, investigator expertise, and patient participation. CEE site locations deliver. The centralized nature of their healthcare systems enables cancer research in a way that other areas cannot match. In addition, while quality is always a vital consideration in oncology studies, CEE sites meet that standard as well and even surpass the quality performance seen in many other site locations.
Resources