Background
A critical step in initiation of a First-In-Human (FIH) study design for a novel therapeutic is determining the appropriate starting dose and dose range to appropriately define the pharmacokinetics (PK), pharmacodynamics (PD) and safety profile. Establishing a precise and predictive PK and PK/PD model is essential to ensure the study proceeds without unnecessary delays in context of the anticipated safety and efficacy from preclinical studies.
Sponsor Problem
A Biotech company faced challenges in accurately translating preclinical dose findings to human studies. The company needed a robust and predictive approach to optimize dose selection and study design and minimize patient risks while ensuring sufficient PD response over the dose escalations. If the dose was not determined correctly, it could have led to setbacks in formulation development, regulatory approvals, and overall study timelines. An inadequate design or dose range risked insufficient data for regulatory approval.
Methods
A range of doses were simulated prospectively to inform the dose escalation scheme. The simulations were critical to inform the study design and to execute the First-In-Human study design. The aim was to provide predicted exposure associated with positive results from preclinical pharmacology studies.
Allucent utilized preclinical data from animal models to derive PK parameters and target an efficacious exposure range. The data were integrated into a predictive model to estimate doses for the First-In-Human study design (Figure 1). The approach involved:
- Building a PK model from preclinical studies to predict human exposure
- Translating biologically active doses from the mouse model to predict human efficacious target exposure
- Validating exposure against observed results to evaluate accuracy and reliability
Solution
Predictive modeling for dose optimization provided the sponsor with confidence in their study design while ensuring regulatory expectations were met. Through the application of this modeling approach, Allucent successfully identified a dose range that aligned with safe human exposure targets. The observed exposure levels in the FIH study fell within the expected range, validating the model’s accuracy. Additionally, the optimized dose escalation strategy enabled the sponsor to obtain pharmacodynamic readouts efficiently. This streamlined approach optimized the number of dose cohorts and study duration, ultimately expediting the drug development process.
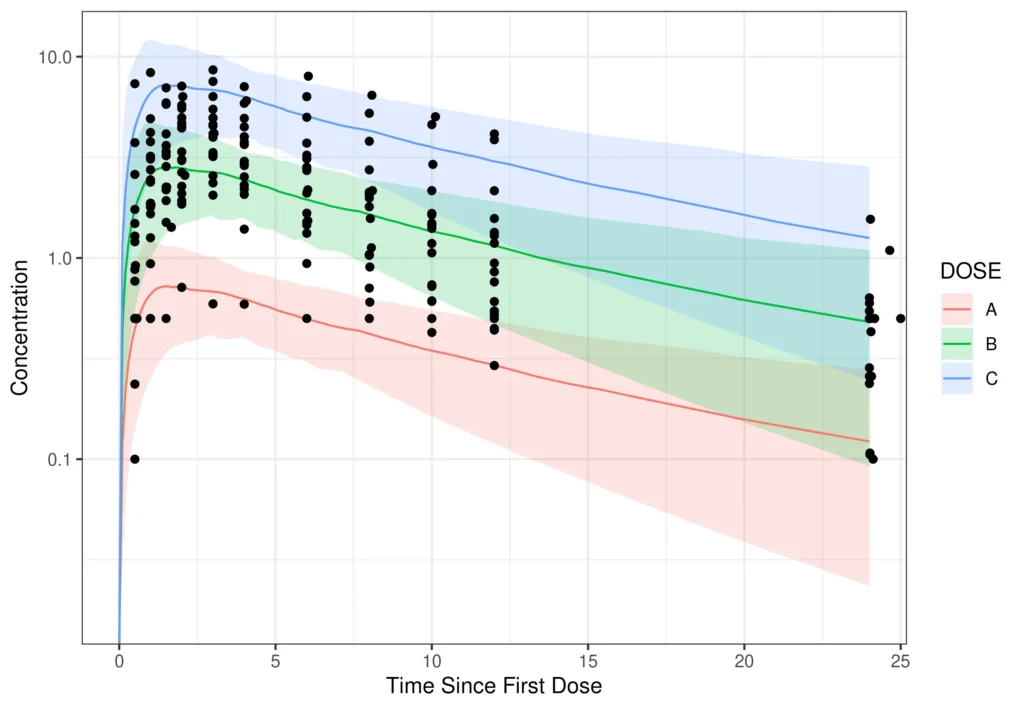
This graph illustrates the predicted drug exposure levels in humans derived from preclinical data (line is median of simulation). The observed human concentrations (black dots) in the FIH study fell within the predicted range (shaded is 90% confidence interval) shown, supporting safe and efficient dose escalation.
By leveraging Allucent’s data-driven methodology, the Biotech company mitigated risks associated with dose miscalculations, ensuring a more seamless transition from preclinical to clinical development. This case underscores the importance of integrating preclinical data with predictive modeling for dose selection to enhance decision-making and accelerate drug development timelines. For a downloadable PDF of this case study, click the link below.