Allergy and Asthma
Strengthen your team with experts who understand the special challenges of allergy studies

From seasonal to perennial allergies, in trials with children and adults, we have the strategic, regulatory, and clinical experience you need.
Allergy treatments encompass a wide variety of allergy types and allergens, each with its own special considerations. Seasonal allergies require extra flexibility to complete trials in the correct time frame. Multiple perennial allergies can happen at once, increasing the challenges in patient recruitment. And trials involving young children and adolescents have specific requirements.
Allucent’s allergy experts have the agility and experience to work effectively within these parameters. We’ve worked on allergy and asthma clinical trials aimed at everything from pet allergies to seasonal and perennial rhinitis. And we’re particularly excited about working on new clinical directions for allergy immunotherapy, with the long-lasting relief this type of treatment can potentially provide.
Wide-Ranging Experience in Allergies
It’s important to choose a team that has the right kind of therapeutic experience for your clinical trials. Our teams have executed studies for allergy treatments across a wide variety of indications:
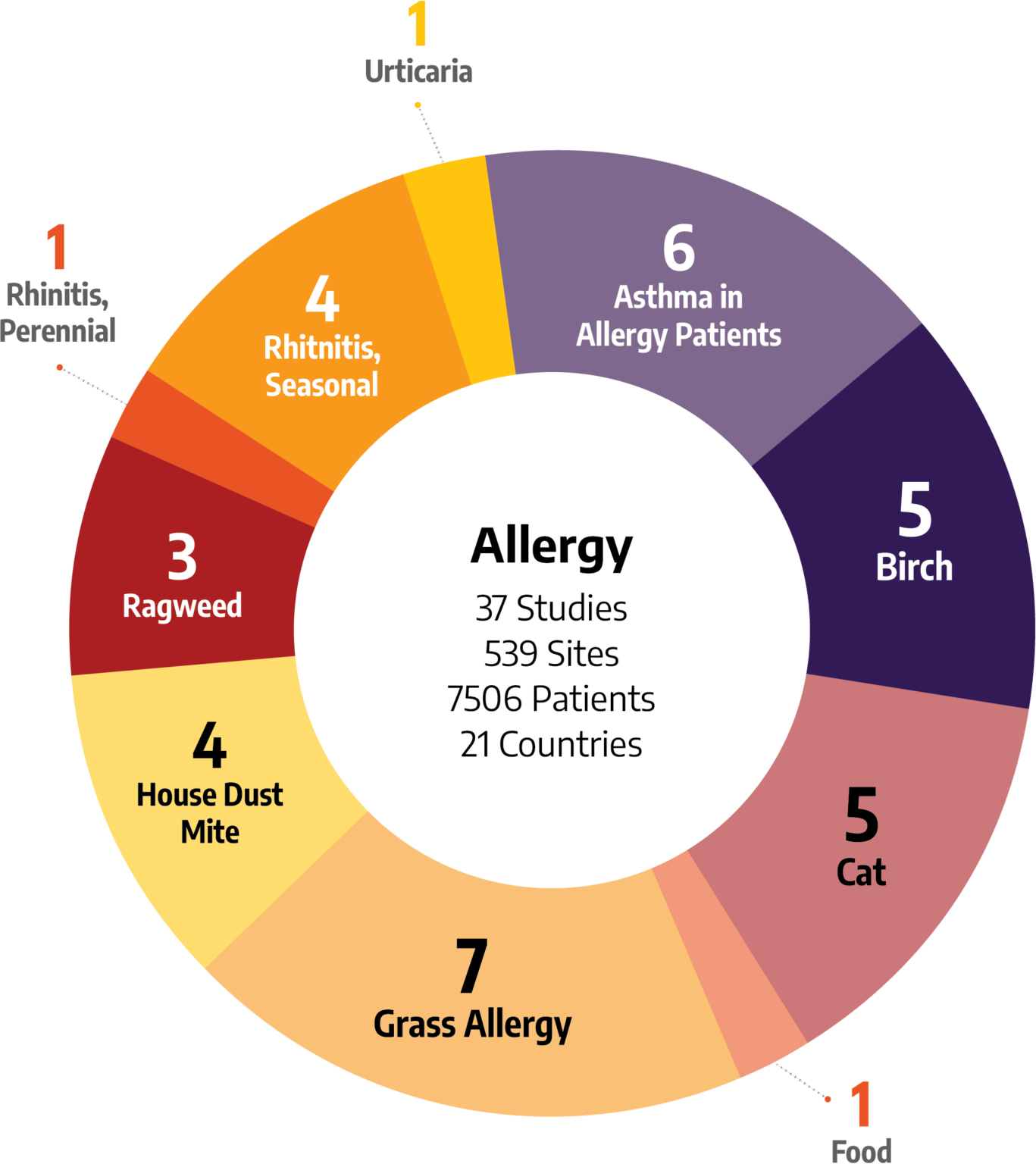
- Birch Allergy
- Cat Allergy
- Food Allergy
- Grass Allergy
- Dust Mite Allergy
- Ragweed Allergy
- Rhinitis, Perennial
- Rhinitis, Seasonal
- Urticaria
Allergy and Asthma Expertise

ALLERGY IMMUNOTHERAPY
Allergy shots decrease sensitivity to allergens and often lead to lasting relief of allergy symptoms even after treatment is stopped. This makes it a modern, beneficial, and cost-effective treatment approach for many people.

PEDIATRIC AND ADOLESCENT
Conducting clinical trials with pediatric and adolescent populations is very different from clinical research with adult populations. Choose an allergy CRO with the experience to overcome these challenges.

SEASONAL ALLERGIES
Successfully completing allergy studies requires more than agility and ownership. For many allergy conditions, seasonality is a factor so studies must be completed within a specific timeframe. Our specialization in conducting allergy clinical trials and dedicated Global Site Activation Unit is built to deliver faster start-up and maximize your available subject recruitment months.
PERENNIAL ALLERGIES
Perennial allergies present their own challenges. Often perennial allergies present with other allergies, so finding patients that present with mono-allergies is critical to determine true efficacy. Our relationships with investigators and allergy experts located around the globe will enable us to recruit the patients needed and make your next allergy study a success.
