Introduction
In April 2023, the European Commission adopted a proposal for a new Directive1 and a new Regulation2 which revise and replace the existing general pharmaceutical legislation in the EU. The proposed change represents the largest reform of the European pharmaceutical legislation in over 20 years. The reforms have major implications for the sector and will be instrumental in shaping the pharmaceutical landscape in the decades to come. In this blog, we summarize some of the key points to look out for with the recent amendments to the proposals from the European Parliament, and what you need to do to prepare ahead of the final sign-off on the legislation.
The New EU Pharma Legislation Reforms: Progress to Date
On the 26th of April 2023, the EU Commission put forward proposals for a new legislative framework for pharmaceuticals in the EU. The proposal adopted by the Commission consisted of the following elements:
- A new Directive: this would repeal and replace Directive 2001/83/EC and Directive 2009/35/EC of the European Parliament and of the Council and incorporating relevant parts of the Paediatric Regulation (Regulation (EC) No 1901/2006)
- A new Regulation: this would repeal and replace Regulation (EC) No 726/2004 and EU Regulation No. 141/2000 on “orphan” medicinal products, and repealing and incorporating relevant parts of the Paediatric Regulation (Regulation (EC) No 1901/2006)
The legislative package included a number of key objectives, among them the aim of creating a single market for medicines in the EU and ensuring better patient access; to maintain Europe’s attractiveness as a location for research and development; to reduce administrative burden for Sponsors; and to put measures in place to foster innovation in antimicrobial resistance (AMR) drug development.
Subsequently, the files were allocated to the Committee on the Environment, Public Health & Food Safety (ENVI) and the appointment of two rapporteurs: Tiemo Wölken for the regulation and Pernille Weiss for the directive. In October 2023 the European Parliament proposed revisions to the Commission’s proposals with key areas of differences including the definition of unmet medical need, length of regulatory data protection, additional incentives for the development of antimicrobials, and removal of the proposed ‘regulatory sandboxes’.
On the 10th of April 2024, the EU Parliament adopted its position on the draft texts 3,4. The proposals are now with the European Council which has not yet adopted its position. Interinstitutional negotiations will be followed up by the newly elected Parliament following the June 2024 elections.
Key Changes to the EU Pharma Legislation
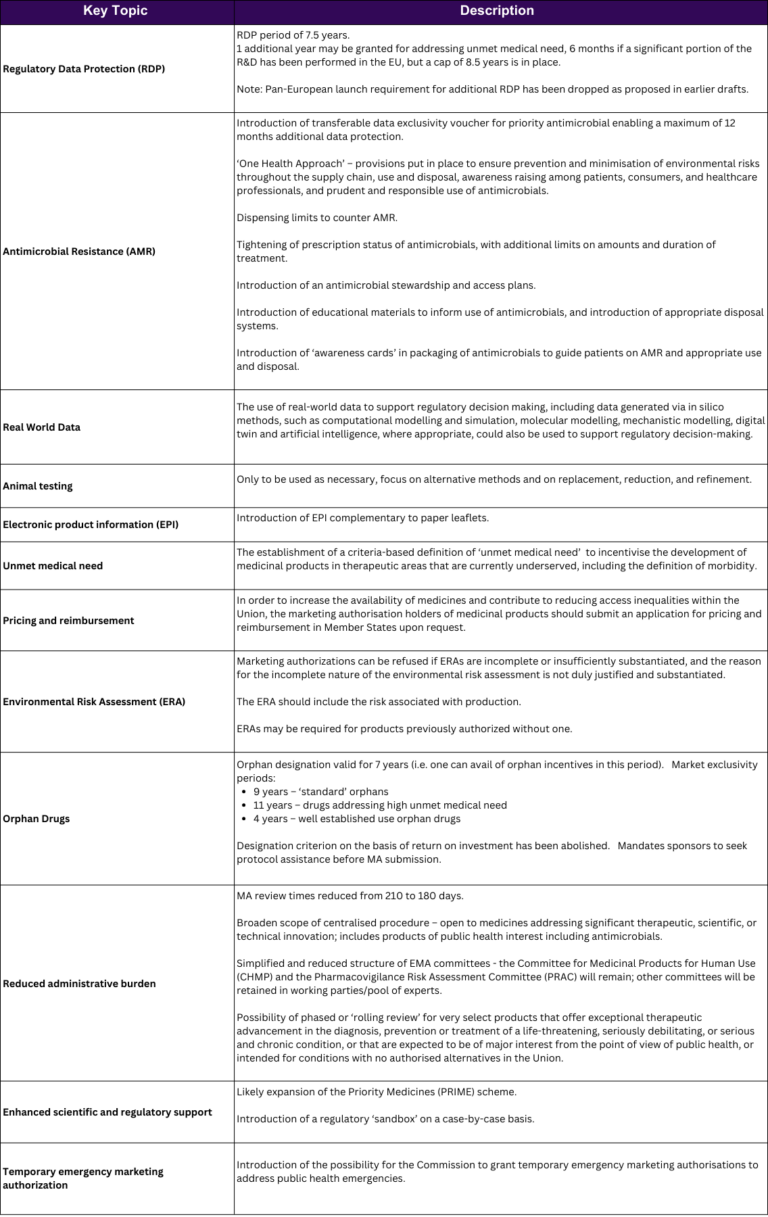
Although there is a way to go before the legislation comes into effect in the EU, with some predicting full implementation as late as 2028, the draft changes as they stand today should be considered when planning future regulatory roadmaps and longer-term planning. A summary of some of the key changes is outlined in the table below. (click on the table to access a printable PDF)
How to Prepare in the EU for the Pharma Legislation Reforms
The aforementioned legislative changes will have far-reaching consequences for many different stakeholders in the pharmaceutical sector, from early-stage biotechs to mature pharma companies. While the legislation implementation timeframe remains unclear, companies should nevertheless begin to scrutinize the draft texts to determine the potential effects on their organizations. GAP analysis should be performed to determine the potential products that may be affected by the changes, what additional regulatory requirements and documentation will need to be put in place, how the manufacturing process may need to be changed, and what resources may be required to implement the changes in a timely manner. Our regulatory and clinical strategy experts at Allucent can guide you through these complex changes and provide cost-effective and scalable tailored solutions for your specific needs. Talk to us today to see how we can help!
If you are interested in hearing more about developing effective regulatory strategies, including gap analyses, listen to our podcast, “Defining the Regulatory Roadmap for Product Success”, by Allucent expert, Merribeth Adams, PhD, Sr. VP Regulatory and Drug Development,
References
- European Commission Proposal for a Directive on the Union code relating to medicinal products for human use. April 2023. Available at: https://health.ec.europa.eu/publications/proposal-directive-union-code-relating-medicinal-products-human-use_en. Last accessed 02 May 2024.
- European Commission for a Proposal for a Regulation laying down Union procedures for the authorisation and supervision of medicinal products for human use and establishing rules governing the European Medicines Agency. April 2023. Available at: https://health.ec.europa.eu/publications/proposal-regulation-laying-down-union-procedures-authorisation-and-supervision-medicinal-products_en. Last accessed 02 May 2024.
- European Parliament legislative resolution of 10 April 2024 on the proposal for a regulation of the European Parliament and of the Council laying down Union procedures for the authorisation and supervision of medicinal products for human use and establishing rules governing the European Medicines Agency, amending Regulation (EC) No 1394/2007 and Regulation (EU) No 536/2014 and repealing Regulation (EC) No 726/2004, Regulation (EC) No 141/2000 and Regulation (EC) No 1901/2006 (COM(2023)0193 – C9-0144/2023 – 2023/0131(COD)). Available at: https://www.europarl.europa.eu/doceo/document/TA-9-2024-0221_EN.html. Last accessed 02 May 2024.
- European Parliament legislative resolution of 10 April 2024 on the proposal for a directive of the European Parliament and of the Council on the Union code relating to medicinal products for human use, and repealing Directive 2001/83/EC and Directive 2009/35/EC (COM(2023)0192 – C9-0143/2023 – 2023/0132(COD)). Available at: https://www.europarl.europa.eu/doceo/document/TA-9-2024-0220_EN.html. Last accessed 02 May 2024.
