Introduction
A successful marketing authorization application is expected to convey the complete story of the drug in a clear, harmonized, and strategic manner. The process is rarely easy, as the authoring process requires distilling the full product development journey, starting from early activities including CMC and nonclinical work and continuing through the output of clinical trials.
Key Benefits of Storyboarding in Marketing Authorization Applications
A marketing authorization application such as a New Drug Application (NDA), Biologics License Application (BLA), or Marketing Authorization Application (MAA) is at its best when tells a coherent story that strategically emphasizes the strengths of the product under review. Storyboarding is a key element in the authoring process that enables a cross-functional team representing each relevant discipline to align on the overall key messages in the submission, and how these messages will be represented and supported in individual modules. The development of the key messages often includes the tailored identification of new data presentations or analyses that will support positioning the product strategically to optimize the chances of regulatory success.
Timing of Storyboarding Activities
A storyboarding process is particularly useful in the months prior to completion of a global or regional marketing application and it is strongly recommended before the statistical analysis plan for the pivotal Phase 3 study is finalized. Early storyboarding activities can begin as early as during preparation for an initial Investigational New Drug (IND) application, and then can be continued and adapted in preparation for the marketing authorization. Recognition and resolution of gaps that weaken the key messages enable a collaborative and structured process that assures that key labeling statements are supported by the most meaningful data outputs presented in a clear and compelling way.
Collaboration and Leadership in the Storyboarding Process
The storyboarding process is typically led by a regulatory affairs representative who has a broad understanding of the product’s development, including the key strategic decisions made throughout. As needed, the storyboarding process should include subject matter experts and authors representing CMC, nonclinical, clinical pharmacology, statistical and clinical disciplines. Once the key messages are collectively aligned agreed upon, it is often helpful for the relevant authors to meet to discuss how to shape the application to construct an optimal narrative supporting regulatory success. To facilitate an efficient storyboarding process, it is recommended to have one or more meeting sessions to bring together relevant parties to discuss the key messages and alignment plans. An active and well-organized storyboarding meeting session enables the representatives from all functions to have their voices heard and often leads to key strategic decisions that ultimately improve the quality of the marketing authorization.
Practical Steps for Effective Storyboarding
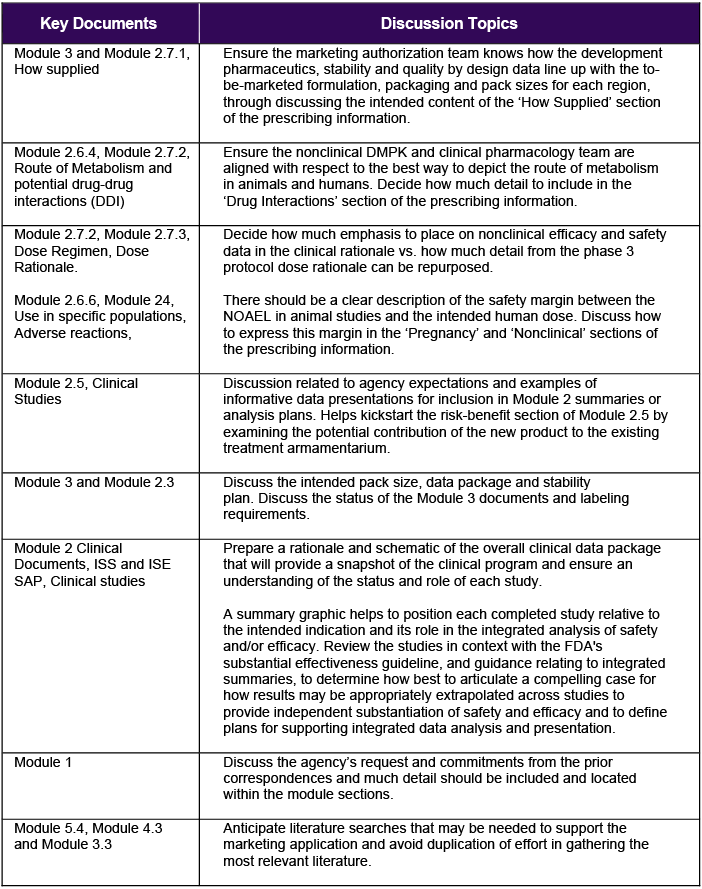
Using all available regulatory guidance, precedent, and experience, a regulatory affairs representative can support a project team by facilitating storyboarding to help align labeling content to the requirements of the specific NDA or BLA module. Table 1 below includes the list of possible discussion topics for storyboarding. The overall aim is for attendees to leave the meeting with a plan of action that includes how information will flow across and between Module 2 summaries or related reports.
Table 1 Storyboarding Discussion Topics
Conclusion
A successful storyboarding process prevents time-consuming and often stressful rework during the subsequent authoring and review of the key documents that will support the marketing application. Discussing the content to support the identified key messages in a proactive, prospective manner instead of recognizing their absence at a late stage enhances the quality of the final application. In conclusion, a comprehensive storyboard that incorporates messaging from all relevant functions, including regulatory, analytical, business development, and marketing, can be the first step in enabling a successful marketing authorization.
At Allucent, we have expertise in helping our sponsors develop effective storyboards that ensure a cohesive and compelling narrative that resonates with regulatory authorities. Learn more about how our A-team of regulatory consulting experts can support you in successfully navigating regulatory hurdles in your product development journey.
