Cell and gene therapies (CGT) have shown tremendous promise as innovative and transformative solutions to address various unmet medical needs. Enthusiasm and interest in this modality continue, with more than 3000 CGT products in development as of the first quarter of 20231. In this first blog, we discuss exosomes — representing a new generation of products with immense therapeutic potential and versatility in their clinical application — and key considerations in their development.
Learn More: What is Cell and Gene Therapy
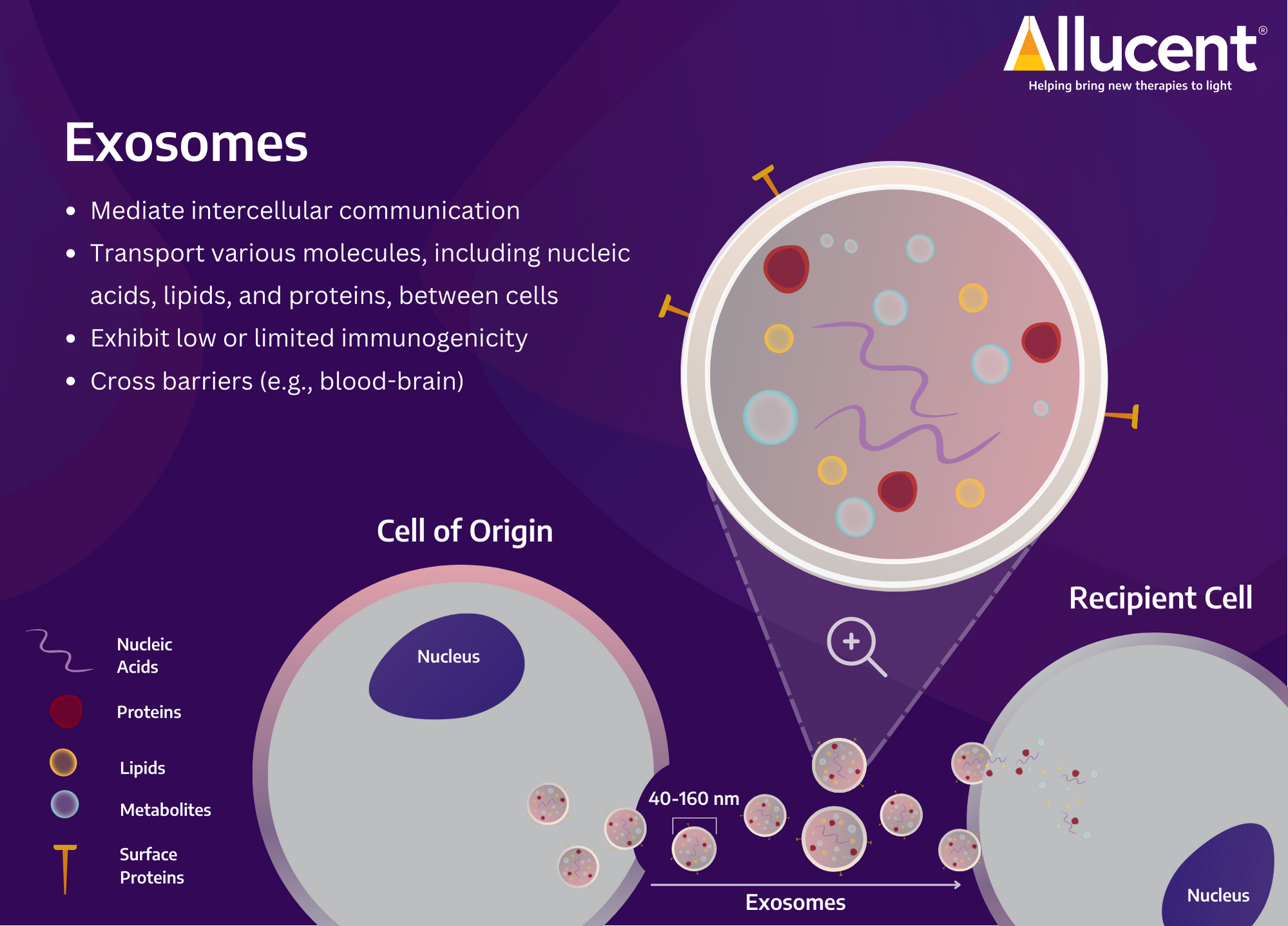
What Are Exosomes
Exosomes (40-160 nm in diameter) are a subtype of extracellular vesicles (EV) capable of carrying a variety of molecules, including nucleic acids, lipids, and proteins2. Released by a range of cell types, exosomes are integral in mediating intercellular communications in normal physiology and pathophysiology2,3. In addition to their ability to shuttle and deliver cargo (locally or at long distances) to the target, circulate, and cross barriers (e.g., blood-brain barrier), exosomes also exhibit low or limited immunogenicity. These features contribute to the growing interest in the clinical application of exosomes2,3.
Therapeutic Potential and Application of Exosomes
According to a published study by Rezaie J et al. in 20224, there were 116 registered clinical trials evaluating exosomes; 58 as biomarkers, 33 as cell-free therapeutic agents, 6 for drug delivery, 2 as cancer vaccines, as well as 17 basic analysis studies for determining exosome kinetics. The clinical applications in these studies cross various indications, including Alzheimer’s disease, neuralgia, osteoarthritis, and cancer4. Although the FDA has not approved any exosome products to date5,6, findings from these clinical trials are expected to improve our understanding of the safety and efficacy of exosomes, as well as provide insights into their long-term viability as a therapeutic modality.
Findings from basic and translational research are also critical in bridging gaps in our understanding of exosomes’ biology, mechanism of action, and safety — to name a few. However, the reliability and reproducibility of such findings depend on the scientific rigor with which the studies are conducted. The International Society for Extracellular Vesicles (ISEV) published an updated guideline in 2018 that underscored the importance of standardization of EV-related activities, from nomenclature to reporting of data7. At the crux of it, ascribing specific function(s) to the appropriate subpopulation of EVs relies not only on their proper identification and careful characterization (e.g., with suitable controls and robust methods) but also on other essential components of quality7. Adequate descriptions of these methods or processes are needed and typically included in regulatory submissions such as Investigational New Drug (IND) applications to the FDA.
Regulatory Considerations for Exosome Products
Key considerations for the development of exosomes and submission of an IND to initiate clinical studies include:
Review Division: the Center for Biologics Evaluation and Research (CBER) at the FDA regulates various product types. Note that communication with CBER’s Product Jurisdiction Officer provides a pathway for sponsors to determine their product classification and the appropriate regulatory jurisdiction8.
CMC/Product Manufacturing Aspect: compliance with CMC regulatory guidelines during the process of product development (e.g., cell cultivation and manipulation, exosome isolation, purification, characterization, and quality control)9,10 to ensure the clinical trial material is a high-quality drug product that will be consistently safe and efficacious is critical to the safety of enrolled subjects and successful interpretation of clinical data. Note that the source of exosomes is also an important regulatory consideration.
Pharmacology, Biodistribution, and Toxicity: leverage findings from adequately designed in vitro and in vivo animal studies. Critical components of the nonclinical program include, but are not limited to:
- understanding of the mechanism of action
- rationale for the dose level and dosing regimen (e.g., dose frequency and duration)
- assessment of therapeutic and adverse effects in relevant animal model(s), and dose-response
- considerations for biodistribution (e.g., tissue distribution, persistence, and fate of exosomes) and tumorigenic potential10-13.
Clinical Objectives: achieving clinical objectives (e.g., safety assessment) in early phase trials requires careful design of the study with considerations for the:
- study population
- treatment plan (e.g., dose and dosing regimen)
- safety evaluation and monitoring
- long-term follow-up plan to understand and mitigate risks associated with delayed adverse events 12,14.
Early Engagement: early communication or interaction with the FDA is highly encouraged, especially for innovative products. One pathway is to engage CBER through an Initial Targeted Engagement for Regulatory Advice (INTERACT) meeting15,16 prior to a pre-IND meeting.
Promising New Frontier in Cell and Gene Therapy (CGT)
Exosomes are expanding the potential of cell and gene therapy. One new frontier of exosome therapy is stem cell-derived exosomes. The interest in this approach is that stem cell-derived exosomes potentially have the therapeutic benefits of a stem cell by its payload of stem cell-derived exocrine factors without the risk of administering live stem cells into the patient. Another new frontier is genetically engineered exosomes, where the exosomes contain genetically engineered membrane proteins. These membrane proteins may allow the targeting of these exosomes to specific tissues.
The promise of such investigational products continues to gain momentum as the field collectively gains experience and answers to key questions — from how do they work to how can they be more targeted? As small to mid-size biopharma companies lead the way in this meaningful endeavor, we understand the substantial effort needed and recognize the commitment required to bring such innovative therapies to patients.
For support in navigating the regulatory complexities of cell and gene therapy product development, including exosome research, Allucent’s A-team is here to partner with you and provide expert guidance. Contact us today.
Allucent brings new therapies to light by solving the distinct challenges of small and mid-size biopharma companies. We’re a global provider of comprehensive drug development and clinical research solutions, including regulatory and drug development consulting, clinical trial operations, biometrics, and clinical pharmacology across a variety of therapeutic areas. Our individualized partnership approach provides experience-driven insights and expertise to assist clients in successfully navigating the complexities of delivering novel treatments to patients. For more details about how the A-Team can support your drug development programs, contact us today.
References
1 American Society of Gene and Cell Therapy. https://asgct.org/global/documents/asgct-citeline-q1-2023-report.aspx.
2 Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020 Feb 7;367(6478):eaau6977.
3 Turturici G, Tinnirello R, Sconzo G, Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am J Physiol Cell Physiol. 2014 Apr 1;306(7):C621-33.
4 Rezaie J, Feghhi M, Etemadi T. Rezaie J, Feghhi M, Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun Signal. 2022 Sep 19;20(1):145.
5 U.S. Food & Drug Administration. Public Safety Notification on Exosome Products. Dec 6, 2019. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/public-safety-notification-exosome-products.
6 U.S. Food & Drug Administration. Consumer Alert on Regenerative Medicine Products Including Stem Cells and Exosomes. Jul 22, 2020. https://www.fda.gov/vaccines-blood-biologics/consumers-biologics/consumer-alert-regenerative-medicine-products-including-stem-cells-and-exosomes.
7 Théry C et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018 Nov 23;7(1):1535750.
8 U.S. Food & Drug Administration. CBER Product Jurisdiction. Mar 26, 2018. https://www.fda.gov/about-fda/center-biologics-evaluation-and-research-cber/cber-product-jurisdiction.
9 U.S. Food & Drug Administration. Guidance for Industry. Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs). Jan 2020. https://www.fda.gov/media/113760/download.
10 U.S. Food & Drug Administration. Guidance for Industry. Drug Products, Including Biological Products, That Contain Nanomaterials. Apr 2022. https://www.fda.gov/media/157812/download.
11 U.S. Food & Drug Administration. Guidance for Industry. Preclinical Assessment of Investigational Cellular and Gene Therapy Products. Nov 2013. https://www.fda.gov/media/87564/download.
12 U.S. Food & Drug Administration. Guidance for Industry. Considerations for the Design of Early-Phase Clinical Trials of Cellular and Gene Therapy Products. Jun 2015. https://www.fda.gov/media/106369/download.
13 U.S. Food & Drug Administration. Guidance for Industry. S12 Nonclinical Biodistribution Considerations for Gene Therapy Products. May 2023. https://www.fda.gov/media/167605/download.
14 U.S. Food & Drug Administration. Guidance for Industry. Long Term Follow-Up After Administration of Human Gene Therapy Products. Jan 2020. https://www.fda.gov/media/113768/download.
15 U.S. Food & Drug Administration. FDA In Brief: FDA Announces Program to Enhance Early Communications With Biological Product Developers. Jun 22, 2018. https://www.fda.gov/news-events/fda-brief/fda-brief-fda-announces-program-enhance-early-communications-biological-product-developers.
16 U.S. Food & Drug Administration. OTAT INTERACT Meeting. Oct 18, 2022. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/otat-interact-meeting.
