
Welcome to our comprehensive three-part blog series where we will seek to guide you through the intricate world of formal meetings between the U.S. Food and Drug Administration (FDA) and sponsors of products that fall under the Prescription Drug User Fee Act (PDUFA). In the drug development lifecycle, regular interactions with the FDA may be beneficial, as they allow the sponsors to seek guidance and recommendations for their product’s development program. Additionally, sponsors gain access to valuable institutional knowledge shared by the FDA, which can help them avoid pitfalls experienced by other development programs, thus helping to ensure their program is best set up for success. In this five-part blog series, we will embark on a journey to unravel the complexities surrounding these pivotal interactions that can play a crucial role in the drug approval process.
For sponsors seeking FDA approval for their innovative therapies, these meetings can be an invaluable source of inspiration and guidance while, at the same time, being a source of apprehension. Our aim is to shed light on the purpose, procedural steps, and preparation for these meetings by providing invaluable insights to empower sponsors with the knowledge they need to navigate this regulatory landscape confidently and maximize their opportunity to engage with the FDA. So, join us as we delve into the core aspects of formal FDA meetings and equip you with the tools to forge ahead in your quest to bring life-changing medications to patients in need.
PART 1- Setting the Stage: An Overview of Formal FDA Meetings and the Initial Steps of the Process
Understanding the different types of FDA meetings, the meeting request procedures, and the subsequent steps is crucial for optimizing these initial interactions. In this first blog in our series, we will provide an overview of these FDA meetings, the different categories of meetings, and best practices for beginning the meeting request process.
Understanding FDA Meeting Types
Under the Prescription Drug User Fee Act (PDUFA), formal meetings offered by the FDA are defined in the most recent FDA guidance document “Formal Meetings Between the FDA and Sponsors or Applicants of PDUFA Products” and fall into one of the following categories:
- Type A: described in the guidance document as “meetings…that are necessary for an otherwise stalled development program to proceed or to address an important safety issue.”
- Type B: meetings to discuss one of six different topics defined in detail in the guidance document.
- Type B (End of Phase [EOP]): meetings held for certain topics at the end of Phase 1, end of Phase 2, or pre-Phase 3.
- Type C: defined in the guidance document as “any meeting other than Type A, Type B, or Type B (EOP) regarding the development and review of a product…”1
These meeting types differ largely in terms of the regulatory purpose of the interaction, the intent and scope of content, and the goal dates for when steps in the process will occur.
Two additional meeting types that were introduced in the PDUFA VII Commitment Letter (found in PDUFA VII: Fiscal Years: 2023-2027) are Type D and INTERACT (INitial Targeted Engagement for Regulatory Advice on CBER/CDER ProducTs).
- Type D: meetings defined as “focused on a narrow set of issues (should be limited to no more than 2 focused topics) and should not require input from more than 3 disciplines or Divisions.”
- INTERACT: Typically, this meeting type occurs before a pre-IND meeting occurs and is used when a sponsor is facing a novel issue that might delay the initiation or progress of IND-enabling studies without the FDA’s early input.2
Although these meetings are not yet reflected in the 2017 Draft Guidance, the FDA has committed to updating this guidance document to include Type D and INTERACT meetings by 30 September 2023 (per FDA’s PDUFA VII Commitment Letter).
Now that we’ve given you an overview of the different meeting types, let’s look at the initial step — requesting a meeting — and some basic information about the process.
Requesting the Meeting
To participate in a formal meeting with the FDA, sponsors must first submit a written request that clearly outlines their drug development program’s strategies, anticipated challenges, approaches to mitigate those challenges, and the relevant questions for the agency. The FDA uses this high-level information to assess the meeting’s necessity, determining whether a meeting should be granted, and which FDA representatives should be involved. Therefore, the written request should be both concise and comprehensive, providing all necessary details without unnecessary verbosity to help ensure that the meeting will be granted.
Meeting Timelines
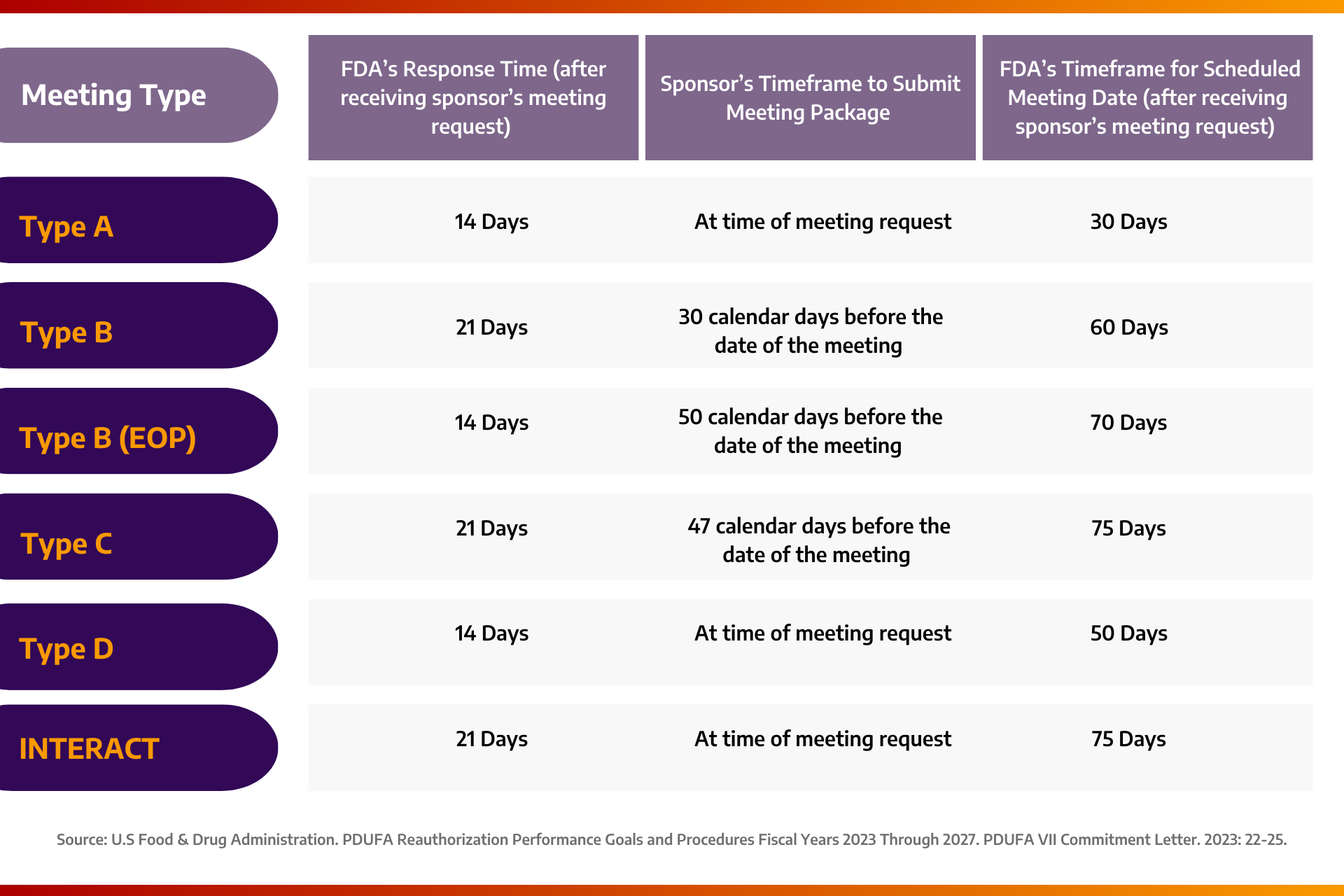
FDA’s Response to the Meeting Request: After reviewing the meeting request, the FDA typically issues a written response, either granting or denying the meeting, within 14 to 21 days. If the meeting is granted, the response will provide the final meeting date, time, and format.
Meeting Package: This is a sponsor-prepared document containing all essential information, rationale, and scientific data required by the FDA to prepare for the meeting. In their response to the meeting request, the FDA will specify the deadline for receiving the meeting package. The meeting package is either due with the meeting request (for Type A, Type D, and INTERACT meetings) or no later than 30 to 50 days before the meeting date for other meeting types.
Meeting Schedule: The FDA aims to schedule meetings within 30 to 75 days after receiving the meeting request, depending on the meeting type. The sponsor’s meeting request should propose potential meeting dates and times, although the final schedule will be determined by the FDA.
Meeting Formats
The FDA offers various meeting formats, including traditional face-to-face (FTF), tele-/videoconferences, and Written Response Only (WRO). Additionally, in the 2023 update on FTF meetings, the FDA announced a new hybrid format that will allow for both in-person and virtual attendees. The desired meeting format should be specified in the sponsor’s meeting request, while the FDA reserves the right to modify it based on their assessment of the meeting’s requirements.
Summary
In this inaugural blog of our 5-part series “Inroads to Approval: Navigating Formal FDA Meeting to Optimize Your Drug Development Program,”, we explored the essential groundwork for engaging in formal FDA meetings. Understanding the different types of FDA meetings, the initial steps in requesting them, and the anticipated timelines is paramount to successfully navigating and utilizing this valuable tool provided by the FDA. These important meetings can play a crucial role in developing a relationship with the FDA, gaining institutional knowledge, avoiding potential pitfalls, and setting your drug development program up for success.
Blog 2 of this series will dive deeper into the intricate process of crafting an effective meeting request and assembling a comprehensive meeting package. We will provide best practices, suggestions, and valuable insights in your pursuit of FDA approval. So, stay tuned for Part 2 where we’ll equip you with the tools to make your meeting requests and packages compelling and concise to best position your program for success and help ensure you receive the most relevant and useful information from your FDA meeting.
For support in navigating formal FDA meetings and optimizing your drug development program, Allucent’s A-team is here to partner with you and provide expert guidance.
Allucent brings new therapies to light by solving the distinct challenges of small and mid-size biopharma companies. We’re a global provider of comprehensive drug development and clinical research solutions, including regulatory and drug development consulting, clinical trial operations, biometrics, and clinical pharmacology across a variety of therapeutic areas. Our individualized partnership approach provides experience-driven insights and expertise to assist clients in successfully navigating the complexities of delivering novel treatments to patients. For more details about how the A-Team can support your drug development programs, contact us today.
References
1U.S Food & Drug Administration. Guidance Document. Formal Meetings Between the FDA and Sponsors or Applicants of PDUFA Products Guidance for Industry. Dec 2017.
2U.S Food & Drug Administration. PDUFA VII Commitment Letter. PDUFA Reauthorization Performance Goals and Procedures Fiscal Years 2023 Through 2027. Accessed 14 August 2023: https://www.fda.gov/media/151712/download?attachment
