Introduction
Central nervous system (CNS) toxicity is a major reason for failure of drugs developed to target this system and others. Therefore, the early detection of potential CNS toxicities is key to inform decision-making in drug development. The assessment of potential CNS toxicities starts with nonclinical studies to understand the mechanism of action, target organ(s) of toxicity, and pharmacokinetic (PK)/toxicokinetic (TK) profile for an investigational medicinal product (IMP). Data from these studies are used to identify hazards (intrinsic toxic properties of an agent) and to characterize risks (probability of a toxicity based on an agent’s exposure and potency) for a product. In order to properly mitigate risks related to a drug’s CNS toxicities, nonclinical data should be thoroughly considered prior to initiation of a first-in-human clinical study.
Types of Nonclinical Data Used in the Evaluation of Therapeutic Products
Allucent uses various types of nonclinical information including pharmacology, safety pharmacology, PK/TK, and toxicology data to understand the risks related to therapeutic products. These data often come from in vitro and in vivo studies and are briefly described below:
Pharmacology:
These studies assess on- and off-target pharmacologic effects of IMPs (see Safety Pharmacology Studies for Human Pharmaceuticals S7A).
Pharmacokinetics (ADME)/Toxicokinetics:
Cmax and AUC are PK/TK parameters used to assess the maximum concentration and overall systemic exposure levels of an IMP, respectively. These parameters may be determined after administration of different doses and across various routes in various species in dedicated PK/TK studies or in safety pharmacology and toxicology studies mentioned below (for further details, see Note for Guidance on Toxicokinetics: The Assessment of Systemic Exposure in Toxicity Studies S3A).
Safety Pharmacology:
These studies investigate potential adverse IMP-related effects on physiological functions at exposure levels in the therapeutic range and above per the ICH S7A guidance document (see Safety Pharmacology Studies for Human Pharmaceuticals S7A). System-specific evaluations of hazards may be done via these studies. For example, CNS safety pharmacology studies typically evaluate motor activity, behavioral changes, coordination, and sensory/motor reflex responses in test species; however, more specialized endpoints are employed in electroencephalography (EEG) studies to detect adverse CNS effects.
Toxicology:
In toxicology studies, drug toxicity is evaluated via observation of body weight, food consumption, clinical signs, anatomical pathology, and clinical pathology. These observations define the no-observed-adverse-effect-level (NOAEL) and can provide evidence of potential CNS toxicities (for more details, see the Guidance on Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals M3(R2)).
Determine if Effect is Drug-Related or Not
Although there are well-characterized drug-related CNS toxicities, there are instances in which seizures, convulsions, and CNS lesions may occur in the absence of treatment. Numerous factors impact susceptibility to these effects including physiological changes, environmental stressors, and age (for examples see Delatte, 2019). Publications have also provided evidence that convulsions may occur spontaneously in species that include rats and dogs (Bielfelt et al., 1971; Edmonds et al., 1979; Satomoto et al., 2012). Therefore, it is important to be able to determine if an observed effect is drug-related or not.
Drug-related effects are typically confirmed when the following occurs:
- Effect occurred in a dose-related manner
- Effect magnitude (i.e., incidence level, severity, or both) increased with incrementally higher doses
- Effect repeated at the same dose level
Determine if Species is Relevant or Not
A number of nonclinical animal models (e.g., monkey, rat, dog) can be used to characterize CNS toxicities of a drug. However, depending on the drug and toxicity in question, certain species may provide data that are not relevant to humans and must be interpreted with caution.
Species relevance is typically established when a test species:
- Expresses the IMP target site
- Exhibits PK profiles for metabolites, protein binding, and exposure that are comparable to that expected in humans
- Presents relevant organ systems that are anatomically and physiologically comparable to that in humans
Determining the Most Sensitive Species
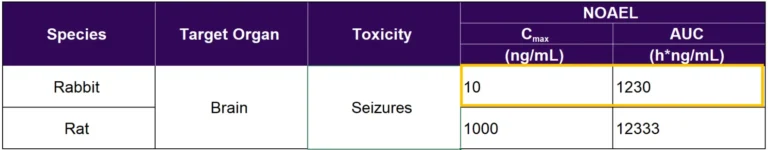
Determining the most sensitive species is critical, as this impacts which data are used to determine safe clinical doses and exposure levels, as well as safety monitoring. Safety margins and monitoring for clinical studies should be based on data from the most sensitive animal species tested. The most sensitive species can be determined based on comparison between exposure levels at the NOAEL (see Table 1) estimated in the species tested. In Table 1, the Cmax and AUC values at the NOAEL in the rabbit are much lower than what is observed in rat, which means the dose-limiting toxicity (seizures) was observed at much lower exposures of the drug in the former. Therefore, the rabbit is the most sensitive species and should be used to determine acceptable clinical dose and exposure levels in humans. Although there are no formal criteria, the FDA typically requires a safety margin of ≥ 10-fold for IMPs that produce severe toxicities (e.g., seizures). Also, in general, the Agency concludes that the most sensitive species is the most relevant, unless justified otherwise.
Table 1. Comparison of Rabbit and Rat NOAEL Exposure Values
Conclusions
Diverse types of data are needed to create a risk profile and to inform the design of clinical studies. Given the numerous factors that may impact the management of risks, collected data must be carefully interpreted to determine if the effects observed are drug related and the species tested are relevant. The identification of drug-related risks positions experts to understand if observed toxicities may be managed in clinical settings and the benefits of treatment outweigh the risks before moving from the nonclinical setting to the clinical setting. Additional information on this topic may be found in this blog “Balancing Act in Preclinical Development: Strategies for Assessing and Managing Neurotoxic Risks in CNS Therapeutics”. To learn more about Allucent’s expertise on risk assessment for novel neurotherapeutics, please check out our recent webinar, “Risk Assessment of Novel Therapeutics: Mechanistic Translation of Preclinical Safety and PK/PD Data to a Clinical Setting”.
Bibliography
Bielfelt SW, Redman HC and McClellan RO (1971) Sire- and sex-related differences in rates of epileptiform seizures in a purebred beagle dog colony. Am J Vet Res 32:2039-2048.
Delatte MS (2019) Issue Resolution Related to Convulsive Profiles in Advanced Issue Resolution in Safety Pharmacology (Mary Jeanne Kallman MKP ed) pp 203-223, Elsevier
Edmonds HL, Jr., Hegreberg GA, vanGelder NM, Sylvester DM, Clemmons RM and Chatburn CG (1979) Spontaneous convulsions in beagle dogs. Fed Proc 38:2424-2428.
Satomoto K, Kuroiwa Y, Masubuchi Y, Uemura H, Oshima Y and Okazaki S (2012) Spontaneous convulsions in Sprague-Dawley rats in carcinogenicity studies. J Toxicol Sci 37:645-647.
