Background: Challenges in First-in-Human Dose Selection
Trispecific T-cell engagers (TCEs) represent a novel class of immuno-oncology therapies. Due to their mechanism of action, which triggers potent immune activation, establishing an appropriate first-in-human (FIH) starting dose is a critical challenge. Traditional approaches rely on the minimum anticipated biological effect level (MABEL), often necessitating ultra-low doses. While conservative, this method can result in numerous cancer patient cohorts receiving subtherapeutic dose levels, delaying the ability to reach clinically meaningful exposures1,2,3,4,5.
The Challenge of Traditional FIH Dose Selection
The conventional MABEL-based methodology frequently produces starting doses that are far below the pharmacologically relevant range. For first-in-class TCEs, this means patients may be exposed to ineffective doses across multiple study cohorts before therapeutic levels are achieved. This inefficiency not only increases development timelines but also burdens patients with limited clinical benefit in early dose-escalation phases.
Solution: A Modified MABEL Strategy for Dose Selection
Allucent applied an integrated translational strategy leveraging:
- Modified MABEL Approach: Identification of a clinically relevant in vitro pharmacology threshold, coupled with pharmacokinetic (PK) modeling using cynomolgus monkey data (Figure 1) for allometric scaling to humans.
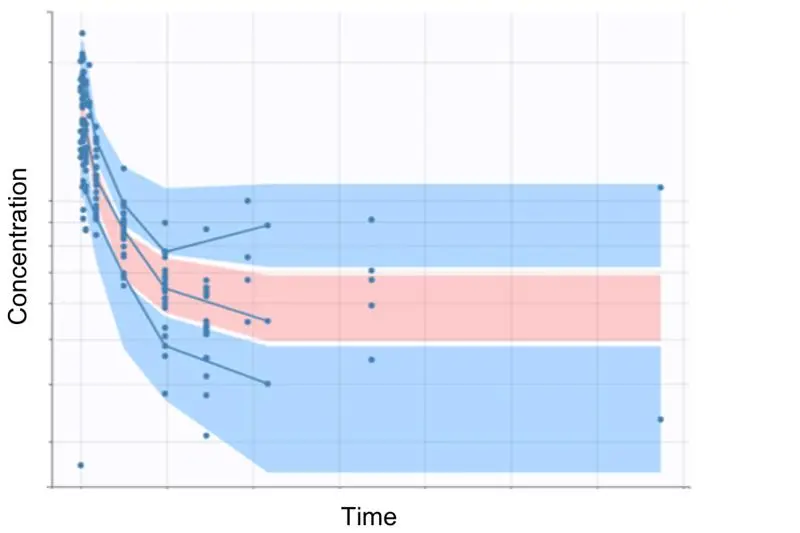
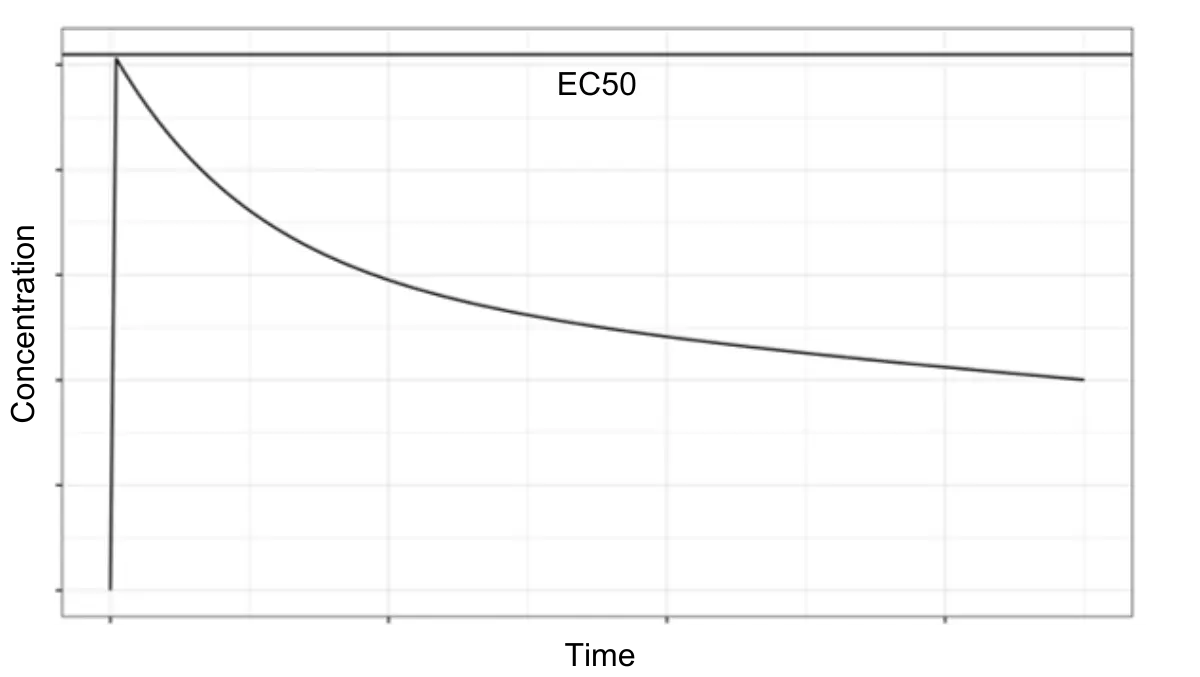
Allucent ensured dose predictions were mechanistically grounded and clinically actionable. This approach provided a higher degree of confidence in selecting a safe, yet meaningful, FIH dose (Figure 2).
Results: Accelerating FIH Trials with Smarter Dose Selection
The modified MABEL approach yielded a first-in-human starting dose more than four times higher than the traditional MABEL calculation. Importantly, the dose fell within four cohorts of the lower end of predicted efficacious exposures, reducing the number of subtherapeutic cohorts and accelerating the path to clinically relevant dosing.
This case demonstrated how modified MABEL strategies can provide sponsors with:
- Faster progression to therapeutic dose ranges
- Improved patient benefit in early cohorts
- Greater efficiency in clinical development timelines
Impact: Smarter Regulatory Dose Selection Strategies
By integrating leveraging the modified MABEL approach, Allucent helped a biotech sponsor advance their novel trispecific TCE into the clinic with a smarter starting dose strategy delivering speed, safety, and stronger translational confidence.
References
1. https://pubmed.ncbi.nlm.nih.gov/28887049/
2. https://pubmed.ncbi.nlm.nih.gov/19177065/
3. https://pubmed.ncbi.nlm.nih.gov/19896825/
4. https://pubmed.ncbi.nlm.nih.gov/20558225/
5. https://ascpt.onlinelibrary.wiley.com/doi/10.1002/cpt.3316
6. https://pmc.ncbi.nlm.nih.gov/articles/PMC9980545/
7. https://ascpt.onlinelibrary.wiley.com/doi/10.1002/cpt.3487?af=R