Lisa Benincosa, PhD
SVP,
Clinical Pharmacology Strategy

Neurological clinical trials face a range of unique challenges due to the complexity of the peripheral and central nervous systems, the intricate nature of neurological diseases, and the difficulty in measuring neurological and psychiatric outcomes. Cognitive impairments, behavioral abnormalities, reduced motor capability, and sensory deficiencies all create a high burden on both patients and caregivers.
As a leading neuroscience CRO, Allucent has supported neuroscience trials for more than 30 years. With over 140 clinical trials in more than 40 indications, we continue to bring hope and breakthrough treatments to countless patients suffering from neurological disorders.

Neurological clinical development requires the specialized services and solutions of a neuroscience CRO to ensure a comprehensive regulatory strategy, a robust study design, effective patient-focused recruitment strategies, and industry-leading technology solutions for successful neuroscience trials.
Allucent Center of Expertise: Neuroscience, offers cross-functional expertise through clinical pharmacology experts, regulatory strategists, biostatistical consultants, medical affairs, and operational experts, who share your deep commitment to developing therapies for neurodegenerative, neurological, rare neurological, pain, and psychiatric conditions to enhance your neuroscience clinical research.
Click icons for more information:
Neurology clinical trials face challenges such as navigating evolving regulatory guidelines, obtaining informed consent in vulnerable populations, and defining meaningful endpoints while managing confounders like the placebo response—key factors for compliance and trial success.
As a specialized neuroscience CRO, Allucent collaborates closely with international regulatory agencies to stay informed on the latest requirements and advancements in neuroscience trials for neurological, pain, and psychiatric treatments. This expertise enables us to navigate the complexities of these trials effectively, including:
Neurology clinical trials and psychiatric clinical trials present a wide range of complex design challenges, from assessing brain penetration for translational PK/PD in dose selection to managing high placebo response in psychiatric and pain indications, necessitating rigorous training and control measures. Neuroscience trials often require multiple subjective assessment scales, demanding standardized and extensive rater training. Additionally, the increasing use of invasive drug administration, fluid biomarker sampling, and specialized imaging further complicates trial execution and design.
As a specialized neuroscience CRO, Allucent brings together clinical pharmacology experts, regulatory strategists, biostatistical consultants, and medical affairs professionals to provide robust and efficient study design recommendations. From first-in-human neurological and psychiatric studies to pivotal trials, our CNS CRO expertise ensures the development of appropriate and clinically meaningful endpoints, dose optimization, and placebo response management. We also support long-term follow-up to maximize the quality and impact of neuroscience clinical research, ultimately leading to better patient outcomes and more effective treatments.
As a leading neuroscience CRO, Allucent has established relationships with site and hospital networks worldwide, bringing extensive expertise in conducting neurology clinical trials and psychiatric clinical trials across all phases of development. Our site networks are equipped to handle complex study requirements, including advanced neuroimaging and neurophysiological monitoring. Partnering with a leading CNS CRO such as Allucent, and collaborating with skilled radiologists and neuroimaging specialists, we deliver high-quality image acquisition, interpretation, and protocol development to support seamless trial execution and reliable data outcomes for neuroscience clinical research.
Ensuring diverse, representative patient populations in neuroscience trials is essential for regulatory compliance but often presents challenges in patient recruitment, particularly for neurology clinical trials and psychiatric clinical trials involving rare neurological conditions. Additionally, many of these trials require prolonged follow-up periods, which can lead to patient dropout over time.
As a leading neuroscience CRO, Allucent’s Dedicated Patient Engagement Team provides tailored strategies to enhance patient recruitment, consent, engagement, diversity, and compliance across neurological, pain, and psychiatric conditions. By collaborating with a leading neurology CRO, like Allucent, we leverage on-site, decentralized, and hybrid trial designs—including DCT approaches—to make patient participation more accessible and less burdensome. We utilize remote assessments, wearables, eCOAs, reliable caregivers, and our extensive CNS CRO site networks to optimize recruitment and retention strategies, ultimately improving the success of neuroscience trials.
As a leading neuroscience CRO, Allucent has established a network of industry-leading preferred partners that provide advanced technology solutions to enhance the design, implementation, and management of neuroscience trials. Our collaborations support critical aspects of trial execution, ensuring high-quality data and streamlined processes.
These solutions include:
Leveraging our extensive partner network and collaborating with a trusted CNS CRO, we ensure efficient collaboration, enhance patient safety, and deliver exceptional data quality for neurology and psychiatric clinical trials.


As a neuroscience CRO, Allucent has conducted a wide range of neuroscience trials, from early phase trials where dose escalation was dependent on repeated CSF sampling and neuroimaging, to worldwide Phase III trials with various interim analyses or adaptive designs.
140+
Studies
1990+
Sites
15500+
Patients
720+
Consulting Projects
All-time years of experience

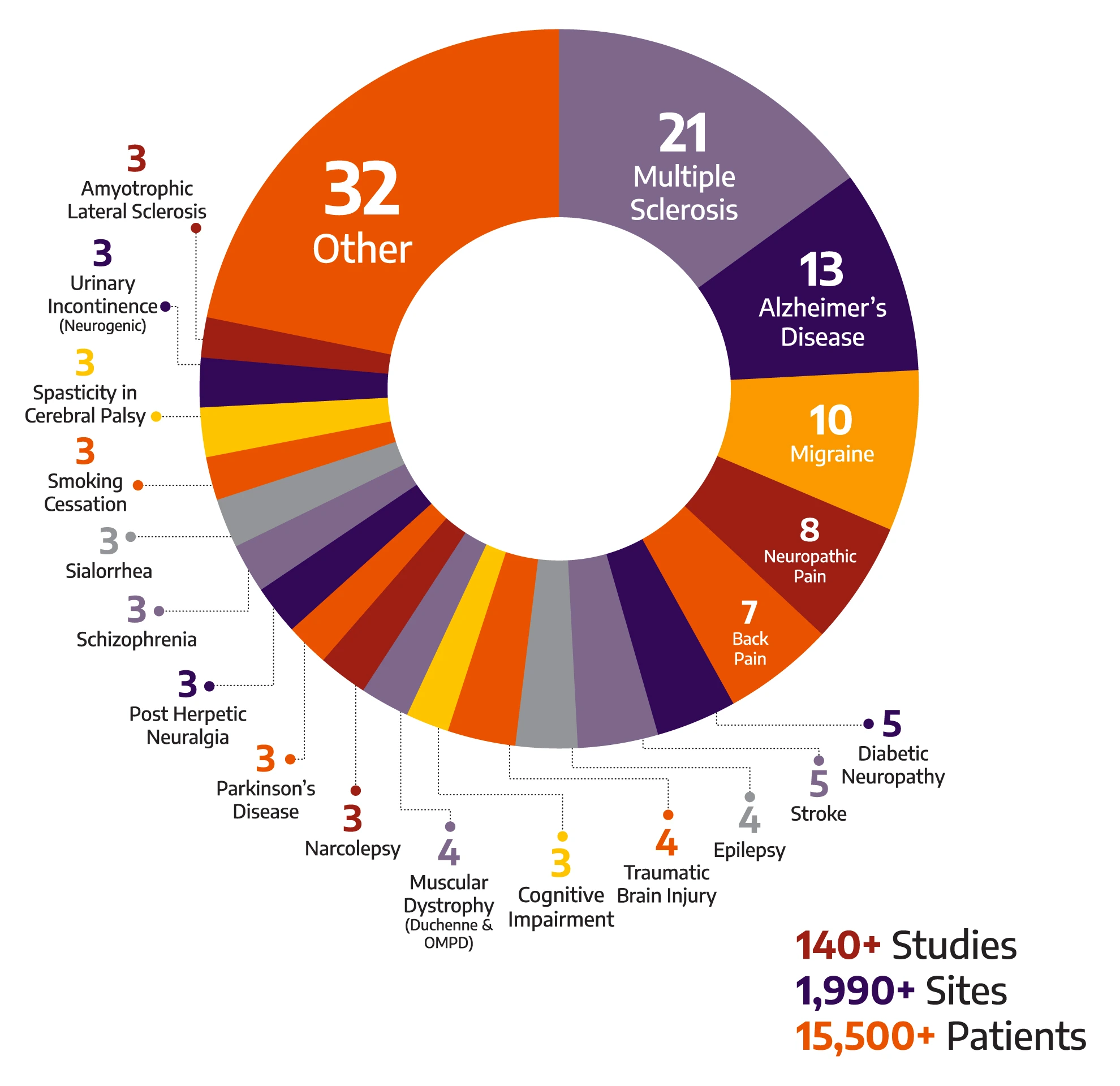
With a global breadth and depth across a wide range of neurology & psychiatry indications, Allucent is a trusted neuroscience CRO partnering with biotech companies to bring innovative therapies to light.




Case Study
Learn More

White Paper
Learn More
Blog
Learn More
Let us know how we can help you bring new therapies to light. Get in touch to get started.
Want to help small and mid-size biopharma companies change the therapeutic landscape?