Navigating Regulatory Challenges: A Collaborative Journey to FDA Approval

Case Study Zachary Swan, PhD (Director, Regulatory Affairs) Background and Challenge Allucent was approached by a commercial-stage biopharmaceutical company focused on infectious diseases to provide writing and strategy support for their unique and complex New Drug Application (NDA) with multiple indications. The company faced a significant challenge when the FDA’s assessment deemed their initial studies in […]
Automated Exploratory Data Analysis to Support Pre-Specified C‑QT Models
Case Study By Payton Woodall (Director, Programming, CPMS) Background Concentration-QT (C-QT) analyses are cost-effective alternatives to thorough QT clinical trials that help establish the drug’s risk of prolonging the QT interval. Prior to conducting a C-QT analysis, several assumptions should be reviewed to ensure the validity of the pre-specified regression approach. Dozens of tabular and […]
Using PBPK to Predict CSF and Brain Exposure of CNS Penetrant Drugs
Case Study By Kai Bartlette (Quantitative Systems Pharmacologist I) Background and Problem Understanding the concentration of drugs in the central nervous system (CNS) is crucial for effective drug development. Traditionally, cerebrospinal fluid (CSF) drug concentrations have served as a surrogate for measuring drug levels in brain tissue, especially for drugs targeting the CNS. Lumbar punctures […]
Unleashing the Power of Predictive Modeling: A Journey to Safe and Effective Dosing for a CNS Drug
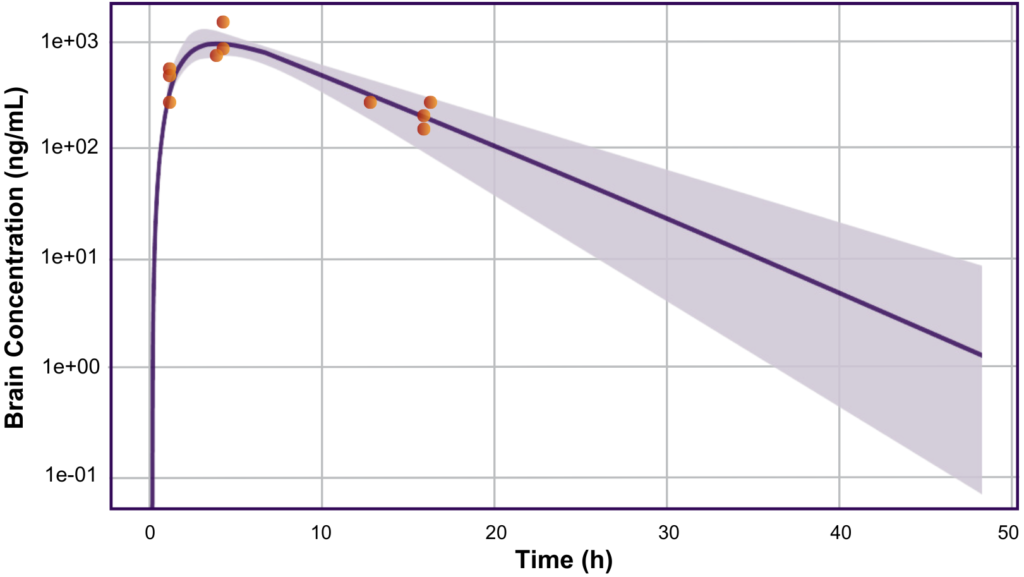
Case Study By Devin Welty (VP, Clinical Pharmacology), Rachel Rozakis (Sr. Clinical Pharmacologist),Marcus Delatte (VP, Regulatory Strategy), and Alison Wakeford (Scientist) Background and Problem Allucent’s client required assistance using modeling and simulation to determine the feasibility and design of a First-in-Human (FIH) study for their program. The client’s drug was created to inhibit an enzyme […]
Using PBPK to Optimize Magnesium Concentrations in an Intravenous Formulation
A new intravenous formulation was developed and the client wanted to know if Allucent could help evaluate the proposed magnesium amount in the new formulation considering various populations of pregnant women, neonates, infants, pediatric patients, adults, and adults with renal impairments. Download the full case study below to learn more. DOWNLOAD RESOURCE
Using PBPK to Extrapolate Animal Tissue Concentrations to Humans
An approved small molecule therapeutic has shown efficacy in numerous target organs of interest in clinical trials. The client wished to show that this efficacy was explained by high drug penetration into these tissues. PBPK modeling can be utilized in multiple stages of drug development. New guidance from regulatory agencies has highlighted key areas for […]
Using Modeling and Simulation to Determine FIH Dose
Allucent’s client requested assistance using modeling and simulation to determine the First-in-Human(FIH) dose for their program. The drug was created to prevent the toxicity of cytotoxic drugs. However, the sponsor felt that the drug could be studied in healthy volunteers in Phase 1, if given carefully and for a short period. Conducting a Phase 1 […]
Gene Therapy

Allucent’s client requested support to bring an AAV gene therapy for a rare neurodegenerative disease into the clinic for a Phase 1b study in patients. Dosescaling from pharmacology and toxicology studies by brain volume alone for administration directly into the brain by MRI-Guided Convection-EnhancedDelivery led to human equivalent doses that lacked a margin of safety. […]
Due Diligence to Support a Client’s Decision-Making
A client needed due diligence performed on the preclinical data, translational dose rationale and first-in-human (FIH) clinical protocol for a central nervous system (CNS) small molecule indicated for a chronic neurodegenerative orphan disease. Download the full case study below to learn more. DOWNLOAD RESOURCE
Complex Concentration-QT Analysis

A Concentration-QT (C-QT) Analysis is a modeling and simulation technique used to help establish the risk of prolonging the QT interval. C-QT analysis can sometimes eliminate the need for a TQT study, which is more expensive and time-consuming. In a C-QT analysis, pharmacokinetic data (concentration of a drug over time) is time matched with EKG […]
