Neurotoxic Risks in Therapeutics: Nonclinical Considerations

By Marcus Delatte, PhD (VP, Regulatory Strategy), Rachel Rozakis, PhD (Sr. Clinical Pharmacologist), Devin Welty, PhD (VP, Clinical Pharmacology), and Alison Wakeford, PhD (Nonclinical Scientist I) Introduction Central nervous system (CNS) toxicity is a major reason for failure of drugs developed to target this system and others. Therefore, the early detection of potential CNS toxicities […]
Using PBPK to Predict CSF and Brain Exposure of CNS Penetrant Drugs
Case Study By Kai Bartlette (Quantitative Systems Pharmacologist I) Background and Problem Understanding the concentration of drugs in the central nervous system (CNS) is crucial for effective drug development. Traditionally, cerebrospinal fluid (CSF) drug concentrations have served as a surrogate for measuring drug levels in brain tissue, especially for drugs targeting the CNS. Lumbar punctures […]
Unleashing the Power of Predictive Modeling: A Journey to Safe and Effective Dosing for a CNS Drug
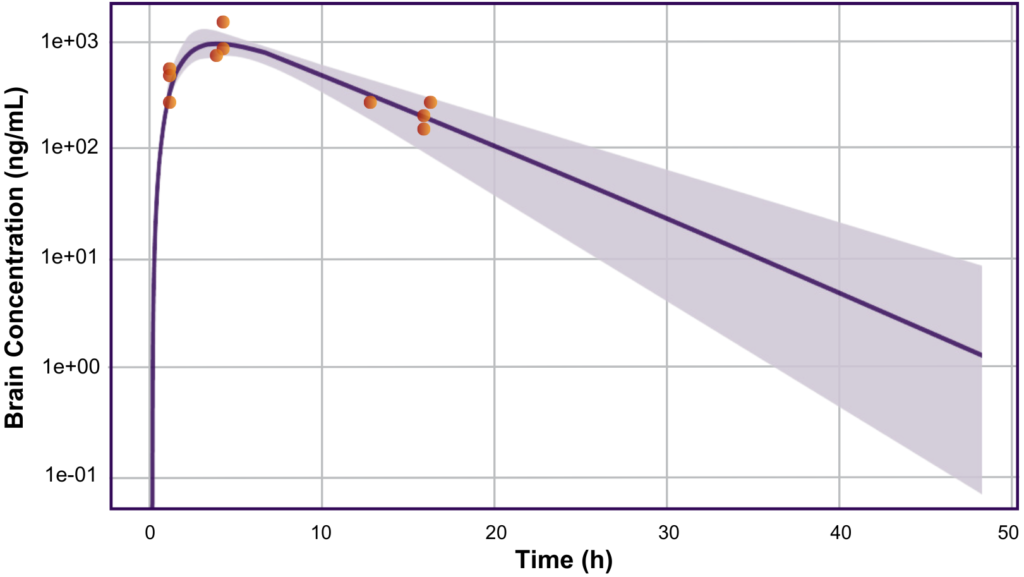
Case Study By Devin Welty (VP, Clinical Pharmacology), Rachel Rozakis (Sr. Clinical Pharmacologist),Marcus Delatte (VP, Regulatory Strategy), and Alison Wakeford (Scientist) Background and Problem Allucent’s client required assistance using modeling and simulation to determine the feasibility and design of a First-in-Human (FIH) study for their program. The client’s drug was created to inhibit an enzyme […]
Balancing Act in Preclinical Development: Strategies for Assessing and Managing Neurotoxic Risks in CNS Therapeutics

By Marcus Delatte (VP, Regulatory Strategy), Alison Wakeford (Scientist), Devin Welty (VP, Clinical Pharmacology), and Rachel Rozakis (Sr. Clinical Pharmacologist) Background Central Nervous System (CNS) disorders are typically severe and may produce various behavioral, anatomical, and physiological changes in millions of patients, which complicates developing effective therapeutics and managing product-related neurotoxicity. Therefore, careful attention should […]
Anti-Depression Medication: Current Landscape and Product Development Trends

Alison Wakeford, PhD, Scientist, Allucent Marcus S. Delatte, PhD, VP Regulatory Strategy, Allucent Background According to the World Health Organization, depression is a common mood disorder, affecting approximately 280 million people globally.1 Depression is characterized by persistent feelings of sadness and loss of interest, occurs across almost all age groups, and manifests in various forms […]
Rare Central Nervous System Disease Trials

Precision medicine offers the promise to treat a variety of conditions, especially rare diseases, with targeted approaches. But developing more personalized medicines can introduce new complexities in the clinical trial process, which can hold back drug developers on the road to commercialization. Find out the factors that make rare neurological diseases a prime target for precision […]
Big passion for small biotech and its potential to address complex diseases
Originally published by Biopharmadive.com Teresa Nunes is responsible for overseeing the activities of the medical affairs and pharmacovigilance teams at the specialized Clinical Research Organization (CRO) leader, Allucent. Here she discusses what drives her passion for helping small and mid-sized biotechnology companies bring new therapies to light for patients around the world – and why she’s […]
Developing CNS Neurotherapeutics with Cerebrospinal PK/PD Clinical Studies
Neurological diseases affect the brain, spinal cord, and peripheral nervous system. They range from diseases that affect large populations such as Alzheimer’s, epilepsy, stroke, Parkinson’s, and multiple sclerosis to rare and genetically inherited diseases like Huntington’s, lysosomal storage diseases, and Amyotrophic Lateral Sclerosis. With more than 2 billion people affected worldwide (and rising), neurological diseases […]
